Research.
Rationally engineering next generation therapies aimed at preventing preterm birth, fostering healthy fetal programming, and improving maternal outcomes,
with the ultimate goal of promoting human health throughout the lifespan. |
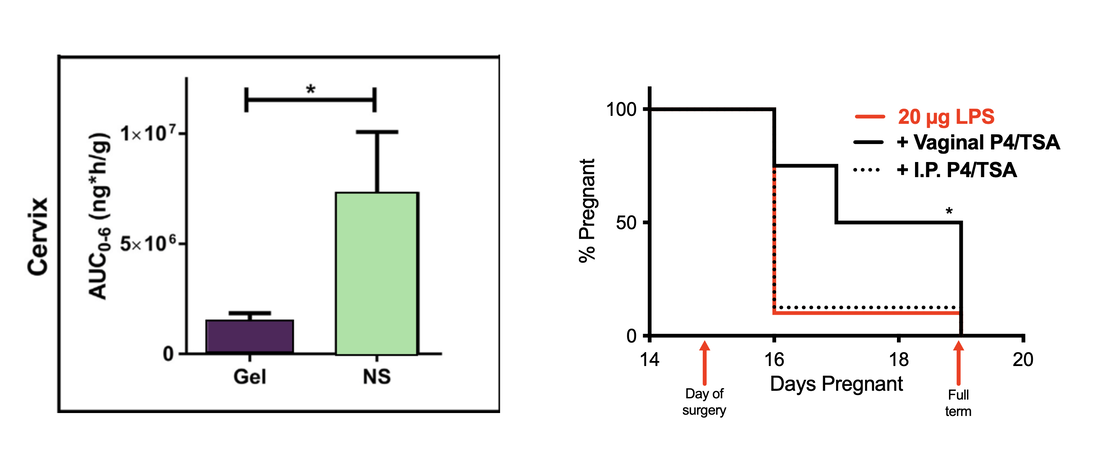
Formulations must be engineered to overcome the vaginal mucus barrier.
|
Vaginal drug administration preferentially targets the female reproductive tract. Bypassing the harsh gastrointestinal environment and hepatic first pass, local drug delivery can lead to more effective therapies. However, these vaginally administered formulations must be engineered to bypass the cervicovaginal mucus barrier, which can both sterically and adhesively trap foreign entities. By rationally engineering therapies to overcome the vaginal mucus barrier, we have identified novel drug candidates for the prevention of inflammation-induced preterm birth. This work highlights the importance of effective drug delivery in understanding drug action in the context of disease.
|
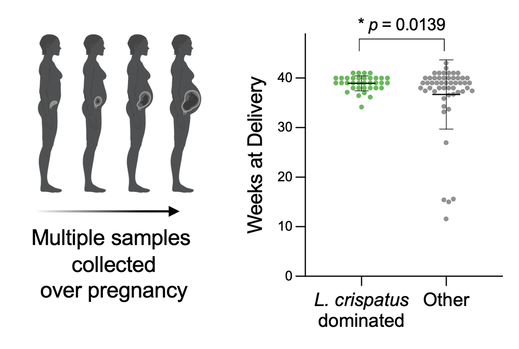
The vaginal microbiome impacts maternal and fetal health outcomes.
Unlike most microbial communities in the human body, an optimal vaginal environment consists of a low level of bacterial diversity, which is typically dominated by Lactobacillus species. Conversely, 30% of women in the U.S. are affected by bacterial vaginosis (BV), an imbalance in the vaginal microbiome marked by a polymicrobial microbiota comprised of anaerobic bacteria, including Gardnerella vaginalis. BV is associated with a higher risk for adverse gynecologic and obstetric outcomes, including sexually transmitted infections, pelvic inflammatory disease, PTB, and fetal brain injury. Bacteria associated with BV cause inflammation, impact levels of proinflammatory cytokines in the female reproductive tract, and impact offspring growth and development. We have observed changes in the time of delivery between women with L. crispatus dominated vaginal microbiota and women with BV. We are interested in exploring how bacterial extracellular vesicles may play a role in these outcomes.
|
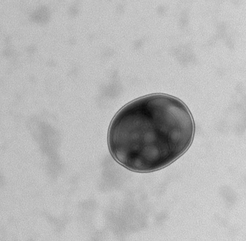
Bacterial extracellular vesicles may mediate host-microbe interactions and serve as a scalable platform for effective vaginal drug delivery.
One possible potentiator of maternal and fetal health outcomes are bacterial extracellular vesicles (bEVs) produced by vaginal microbes. bEVs are bacterially-derived nanoparticles which enable host-microbe communications and impact immune responses. bEVs are spontaneously produced by both gram-positive and gram-negative bacteria, and facilitate horizontal gene transfer, defense against the host immune system, and transport of virulence factors. Establishing the role of vaginal microbe-derived bEVs in maternal and fetal health will serve as the foundation for engineering bEVs as therapeutics. As bacteria are easily manipulated and easily cultured, they present an ideal candidate for manufacturing biocompatible therapies for improving birth outcomes, minimizing postpartum complications, and mediating beneficial prenatal programming.
|
Publications
Check out our Google Scholar and PubMed profiles for the most up-to-date publications!
- Extracellular vesicles as carriers of mRNA: Opportunities and challenges in diagnosis and treatment
Kirian RD, Steinman D, Jewell CM, Zierden HC. Theranostics March 2024.
- Cervicovaginal mucus barrier properties during pregnancy are impacted by the vaginal microbiome
Zierden HC, DeLong K, Zulfiqar F, Ortiz Ortiz J, Laney V, Bensouda S, Hernández N, Hoang TM, Lai SK, Hanes J, Burke AE, Ensign LM. , Frontiers in Cellular and Infection Microbiology March 2023. - Extracellular vesicles are dynamic regulators of maternal glucose homeostasis during pregnancy
Zierden HC, Marx-Rattner R, Rock KD, Montgomery KR, Anastasiadis P, Folts L, Bale TL., Nature Scientific Reports March 2023.
- Next generation strategies for preventing preterm birth
Zierden HC, Shapiro RL, DeLong K, Carter D, Ensign LM. , Advanced Drug Delivery Reviews July 2021: Vol. 174, 190-209. - Avoiding a sticky situation: bypassing the mucus barrier for improved local drug delivery
Zierden HC, Josyula A, Shapiro RL, Hsueh HT, Hanes J, Ensign LM. Trends in Molecular Medicine. - Enhanced drug delivery to the reproductive tract using nanomedicine reveals therapeutic options for prevention of preterm birth
Zierden HC, Ortiz Ortiz JI, DeLong K, Yu J, Li G, Dimitrion P, Bensouda S, Laney V, Bailey A, Anders NM, Scardina M, Mahendroo M, Mesiano S, Burd I, Wagner G, Hanes J, Ensign LM. Science Translational Medicine 13 Jan 2021: Vol. 13, Issue 576, eabc6245. - The cervicovaginal mucus barrier to HIV-1 is diminished in bacterial vaginosis
Hoang TM, Toler E, DeLong K, Mafunda NA, Bloom SM, Zierden HC, Moench TR, Coleman JS, Hanes J, Kwon DS, Lai SK, Cone RA, Ensign LM. PLOS Pathogens. 2020;16(1):e1008236. - Characterization of an adapted murine model of intrauterine inflammation-induced preterm birth
Zierden HC, Ortiz Ortiz JI, Dimitrion P, Laney V, Bensouda S, DeLong K, Hanes J, Ensign LM. Am J Pathol. 2020; 190(2):295–305. - Conceptual Design of a Universal Donor Screening Approach for Vaginal Microbiota Transplant
Kevin DeLong, Sabrine Bensouda, Fareeha Zulfiqar, Hannah C Zierden, Thuy M Hoang, Alison G Abraham, Jenell S Coleman, Richard A Cone, Patti E Gravitt, Craig Walter Hendrix, Edward J Fuchs, Charlotte A Gaydos, Ethel D Weld, Laura M Ensign. Front Cell Infect Microbiol. 2019 Aug 28;9:306. - Mucus-penetrating budesonide nanolususpension enema for local treatment of inflammatory bowel disease
Date AA, Halpert G, Babu T, Ortiz J, Kanvinde P, Dimitrion P, Narayan J, Zierden H, Betageri K, Musmanno O, Wiegand H, Huang X, Gumber S, Hanes J, Ensign LM. Biomaterials. 2018 Dec; 185:97-105. - Development of a mucoinert progesterone nanosuspension for safer and more effective prevention of preterm birth
Hoang TM, Zierden HC, Date A, Ortiz J, Anders N, He P, Hanes J, Mahendroo M, Ensign LM. Journal of Controlled Release. 2018 Dec; 295:74-86.